Amino acids are the monomers that are the building blocks of proteins. Proteins serve as structural support inside the cell and they perform many vital chemical reactions. A chain of amino acids is called a polypeptide. Each protei is a molecule made up of different combinations of 20 types of smaller, simpler amino acids. Protein molecules are long chains of amino acids that are folded into a three-dimensional shape.
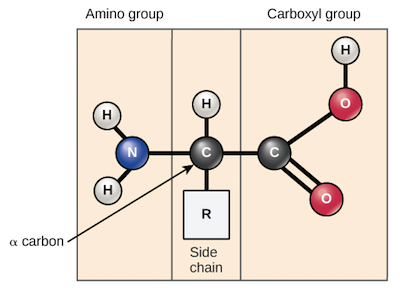
Amino acids share a basic structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group (), a carboxyl group (), and a hydrogen atom.
Although the generalized amino acid shown above is shown with its amino and carboxyl groups neutral for simplicity, this is not actually the state in which an amino acid would typically be found. At physiological pH (7.2 - 7.4), the amino group is typically protonated and bears a positive charge, while the carboxyl group is typically deprotonated and bears a negative charge.
Every amino acid also has another atom or group of atoms bonded to the central atom, known as the R group, which determines the identity of the amino acid. For instance, if the R group is a hydrogen atom, then the amino acid is glycine, while if it’s a methyl () group, the amino acid is alanine. The twenty common amino acids are shown in the chart below, with their R groups highlighted in blue.

A few other amino acids have R groups with special properties:
- Proline has an R group that’s linked back to its own amino group, forming a ring structure. This makes it an exception to the typical structure of an amino acid, since it no longer has the standard amino group. The odd ring structure is because proline often causes bends or kinks in amino acid chains.
- Cysteine contains a thiol (-SH) group and can form covalent bonds with other cysteines.
Finally, there are a few other “non-canonical” amino acids that are found in proteins only under certain conditions.
Translation of Amino Acids
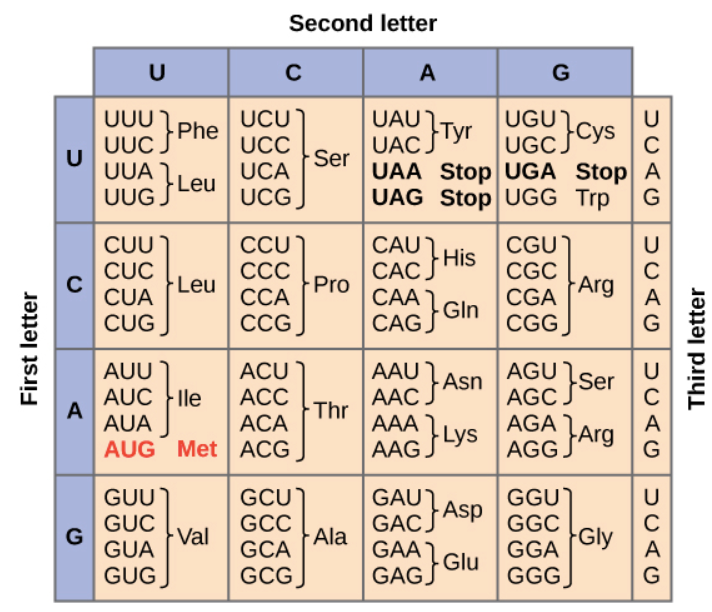
During translation, which is the second major step in gene expression, the mRNA is “read” according to the genetic code, which relates the DNA sequence to the amino acid sequence in proteins. Each group of three bases in mRNA constitutes a codon, and each codon specifies a particular amino acid (hence, it is a triplet code). The mRNA sequence is thus used as a template to assemble—in order—the chain of amino acids that form a protein.