Specialized lipids called phospholipid are major components of the plasma membrane. Like fats, they are typically composed of fatty acid chains attached to a backbone of glycerol. Instead having three fatty acid tails, however, phospholipids generally have just two, and the third carbon of the glycerol backbone is occupied by a modified phosphate group. Different phospholipids have different modifiers on the phosphate group, with choline (a nitrogen-containing compound) and serine (an amino acid) being common examples. Different modifiers give phospholipids different properties and roles in a cell.
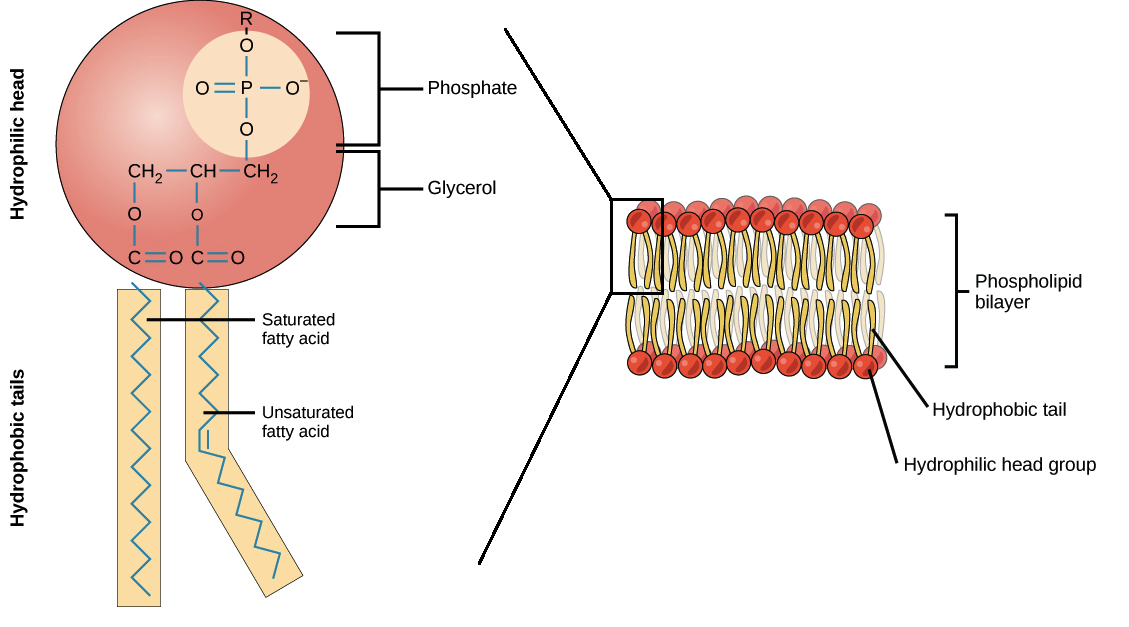
A phospholipid is an amphipathic molecule, meaning it has a hydrophobic part and a hydrophilic part. The fatty acid chains are hydrophobic and do not interact with water, whereas the phosphate-containing group is hydrophilic (because of its charge) and interacts readily with water. In a membrane, phospholipids are arranged into a structure called a bilayer, with their phosphate heads facing the water and their tails pointing towards the inside (above). This organization prevents the hydrophobic tails from coming into contact with the water, making it a low-energy, stable arrangement.
If a drop of phospholipids is placed in water, it may spontaneously form a sphere-shaped structure known as a micelle, in which the hydrophilic phosphate heads face the outside and the fatty acids face the interior of this structure. Formation of micelle is an energetically favored because it sequesters the hydrophobic fatty acid tails, allowing the hydrophilic phosphate head group to instead interact with the surrounding water.