An unsaturated fat is a type of lipid and is a fat in which there is at least one double bond within the fatty acid chain (unliked saturated fats). A fatty acid chain is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond.
The double bonds in unsaturated fatty acids, like other types of double bonds, can exist in either a cis or a trans configuration. In the cis configuration, the two hydrogens associated with the bond are on the same side, while in a trans configuration, they are on opposite sides (see below). A cis double bond generates a kink or bend in the fatty acid, a feature that has important consequences for the behavior of fats.
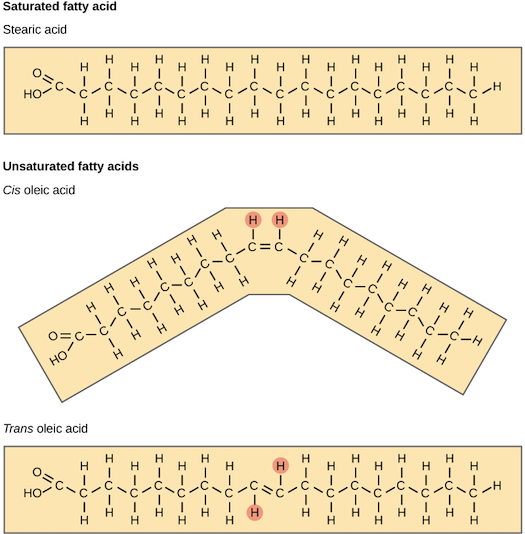
Saturated fatty acids tails are straight, so fat molecules with fully saturated tails can pack tightly against one another. This tight packing results in fats that are solid at room temperature (have a relatively high melting point). For instance, most of the fat in butter is saturated fat.
In contrast, cis-unsaturated fatty acid tails are bent due to the cis double bond. This makes it hard for fat molecules with one or more cis unsaturated fatty acid tails to pack tightly. So, fats with unsaturated tails tend to be liquid at room temperature (have a relatively low melting point) – they are what we commonly call oils. For instance, olive oil is mostly made up of unsaturated fats.