Monomers and Polymers
Most large biological molecules are polymers, long chains made up of repeating molecular subunits, or building blocks, called monomers. Carbohydrates, nucleic acids, and proteins are often found as long polymers in nature. Lipids are not usually polymers and are smaller than the other three.
Dehydration Synthesis
Large biological molecules often assemble via dehydration synthesis reactions, in which one monomer forms a covalent bond to another monomer (or growing chain of monomers), releasing a water molecule in the process. You can remember what happens by the name of the reaction: dehydration, for the loss of the water molecule, and synthesis, for the formation of a new bond.
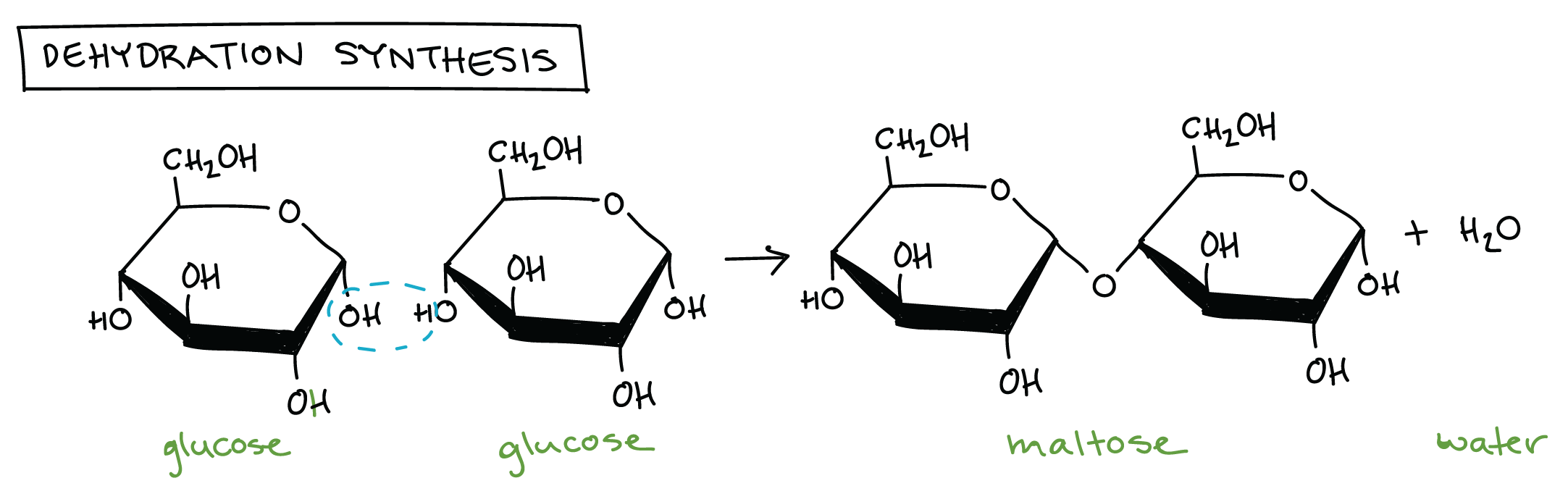
In the dehydration synthesis reaction above, two molecules of the sugar glucose (monomers) combine to form a single molecule of the sugar maltose. One of the glucose molecules loses an H, the other loses an OH group, and a water molecule is released as a new covalent bond forms between the two glucose molecules. As additional monomers join by the same process, the chain can get longer and longer and form a polymer.
Even though polymers are made out of repeating monomer units, there is lots of room for variety in their shape and composition. Carbohydrates, nucleic acids, and proteins can all contain multiple different types of monomers, and their composition and sequence is important to their function.
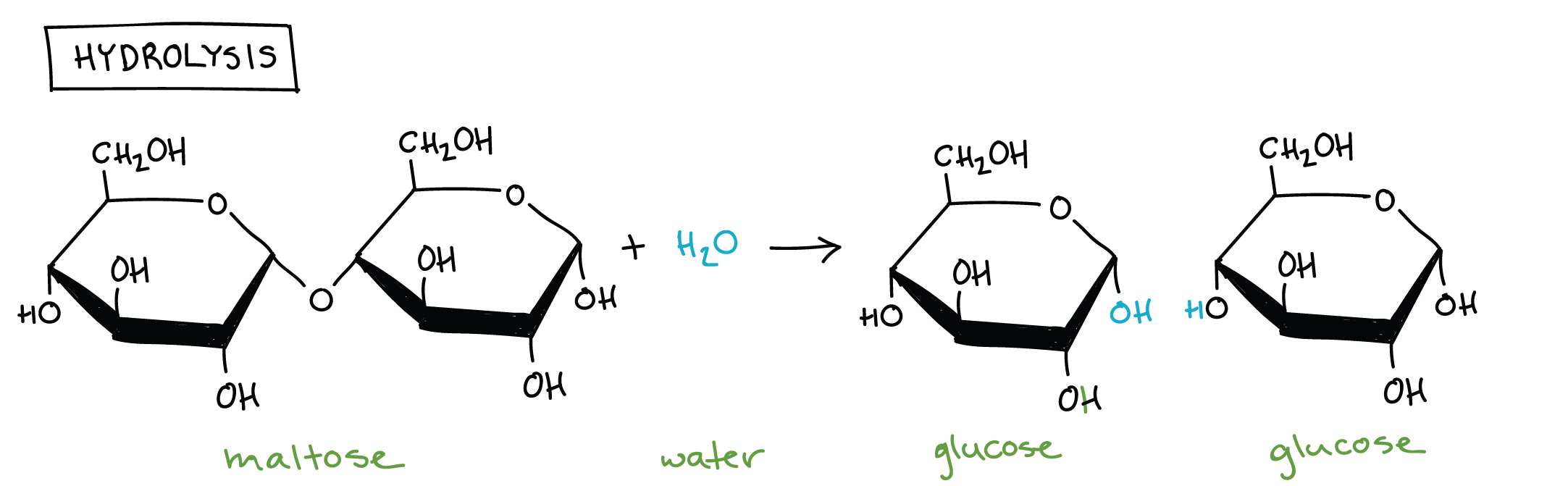
Hydrolysis
Polymers are broken down into monomers via hydrolysis reactions, in which a bond is broken, or lysed, by addition of a water molecule.
During a hydrolysis reaction, a molecule composed of multiple subunits is split in two: one of the new molecules gains a hydrogen atom, while the other gains a hydroxyl (-OH) group, both of which are donated by water. This is the reverse of a dehydration synthesis reaction, and it releases a monomer that can be used in building a new polymer. For example, in the hydrolysis reaction below, a water molecule splits maltose to release two glucose monomers. This reaction is the reverse of the dehydration synthesis reaction shown above.
Dehydration synthesis reactions build molecules up and generally require energy, while hydrolysis reactions break molecules down and generally release energy. Carbohydrates, proteins, and nucleic acids are built up and broken down via these types of reactions, although the monomers involved are different in each case.
In the body, enzymes catalyze, or speed up, both the dehydration synthesis and hydrolysis reactions. Enzymes involved in breaking bonds are often given names that end with -ase: for instance, the maltase enzyme breaks down maltose, lipases break down lipids, and peptidases break down proteins.