Introduction to the Properties of Water
Water is a common substance in the bodies of organisms and has some unusually chemical properties that make it very good at support life. These properties are important to biology on many different levels, from cells to organisms to ecosystems.
- Solvent properties of water. Water can dissolve many polar and charged molecules.
- Cohesion and adhesion of water. Water can stick to itself (cohesion) and other molecules (adhesion).
- Specific heat, heat of vaporization, and density of water. Water has a high heat capacity and heat of vaporization, and ice—solid water—is less dense than liquid water.
Water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with each other and with other molecules.
Polarity of Water Molecules
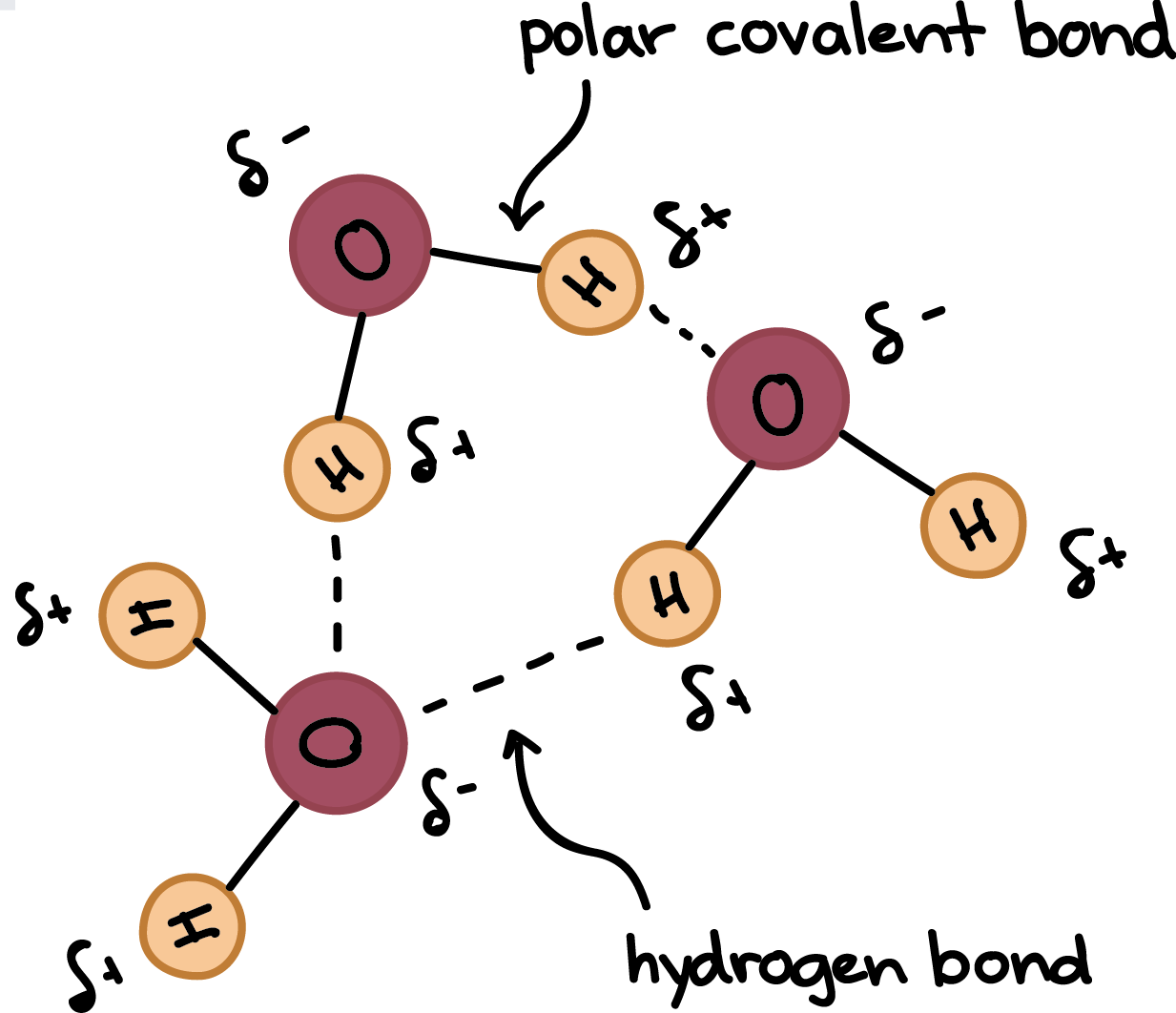
The key to understanding water’s chemical behavior is its molecular structure. A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. All of the electron pairs—shared and unshared—repel each other.
The most stable arrangement is the one that puts them farthest apart from each other: a tetrahedron, with the bonds forming out of the four “legs.” The lone pairs are slightly more repulsive than the bond electrons, so the angle between the bonds is slightly less than the 109° of a perfect tetrahedron, around 104.5°.
Because oxygen is more electronegative than hydrogen, the atom hogs electrons and keeps them away from the atoms. This gives the oxygen end of the water molecule a partial negative charge, while the hydrogen end has a partial positive charge. Water is classified as a polar molecule because of its polar covalent bonds and its bent shape.
Hydrogen Bonding of Water Molecules
Thanks to their polarity, water molecules attract each other. The positive end of one—a hydrogen atom—associates with the negative end of another—an oxygen atom.
These attractions are an example of hydrogen bonds, weak interactions that form between a hydrogen with a partial positive charge and a more electronegative atom, such as oxygen. The hydrogen atoms involved in hydrogen bonding must be attached to electronegative atoms, such as , , or .
Water molecules are also attracted to other polar molecules and to ions. A charged or polar substance that interacts with and dissolves in water is said to be hydrophilic. In contrast, nonpolar molecules like oils and fats do not interact well with water. They separate from it rather than dissolve in it and are called hydrophobic.