Diastereomers are any stereoisomers that are not enantiomers. One common example of a diastereomer is a cis-trans isomer. Cis-trans isomers can occur when atoms or functional groups are situated on either end of a rigid carbon-carbon bond, such as a double bond. In this case, restricted rotation about the double bond means that the atoms or groups attached to either end can exist in one of two possible configurations. If either carbon is attached to two of the same atoms or groups, then this won’t matter; however, if both carbons are attached to two different atoms or functional groups, then two different arrangements are possible.
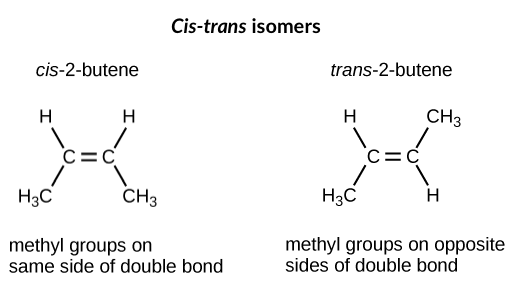
For example, in 2-butene (), the two methyl groups () can occupy different positions relative to the double bond central to the molecule. If the methyl groups are on the same side of the double bond, this is called the cis configuration of 2-butene; if they are on opposite sides, this is the trans configuration.
In the trans configuration, the carbon backbone is more or less linear, whereas in the cis configuration, the backbone contains a bend, or kink. (Some ring-shaped molecules can also have cis and trans configurations, in which attached atoms are trapped on the same or on opposite sides of the ring, respectively)