Disaccharide (di- = “two”) is a type of carbohydrate and forms when two monosaccharides join together via dehydration synthesis. In this process, the hydroxyl group of one monosaccharide combines with the hydrogen of another, releasing a molecule of water and forming a covalent bond known as a glycosidic linkage.
For instance, the diagram below shows glucose and fructose monomers combining via a dehydration reaction to form sucrose, a disaccharide we know as table sugar. (The reaction also releases a water molecule, not pictured.)
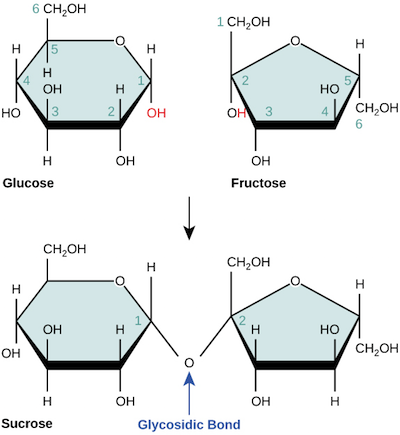
In some cases, it’s important to know which carbons on the two sugar rings are connected by a glycosidic bond. Each carbon atom in a monosaccharide is given a number, starting with the terminal carbon closest to the carbonyl group (when the sugar is in its linear form). This numbering is shown for glucose and fructose, above. In a sucrose molecule, the carbon of glucose is connected to the carbon of fructose, so this bond is called a - glycosidic linkage.
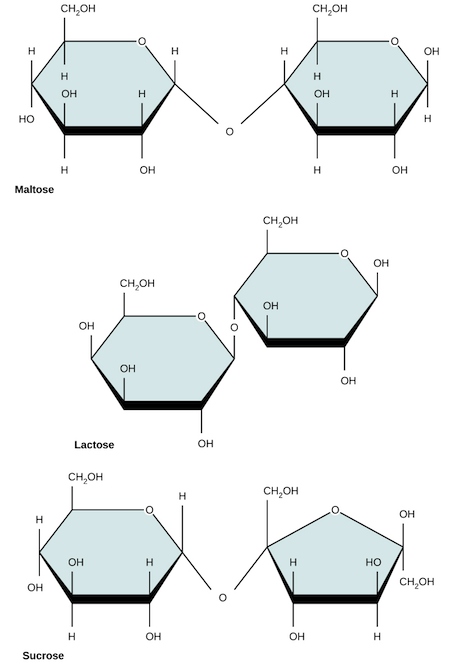
Common disaccharides include lactose, maltose, and sucrose. Lactose is a disaccharide consisting of glucose and galactose and is found naturally in milk. Many people can’t digest lactose as adults, resulting in lactose intolerance (which you or your friends may be all too familiar with). Maltose, or malt sugar, is a disaccharide made up of two glucose molecules. The most common disaccharide is sucrose (table sugar), which is made of glucose and fructose.