Enantiomers are a type of isomer, specifically stereoisomers that are non-superimposable mirror images of each other (“non-superimposable” means that the two molecules cannot be perfectly aligned one on top of the other in space). Enantiomerism is often seen in molecules containing one or more asymmetric carbons, which are carbon atoms that are attached to four different atoms or groups.
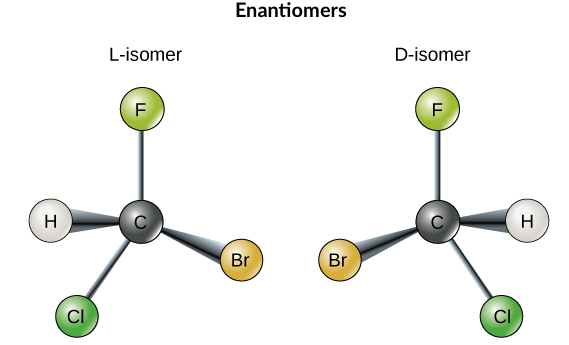
The molecules above are an example of an enantiomer pair. Both have the same molecular formula and are made up of a chlorine, a fluorine, a bromine and a hydrogen atom bonded to a central carbon atom. However, the two molecules are mirror images of one another, and if you try to place them on top of each other, you’ll find that there’s no way to make them fully line up. Enantiomers are often compared to a person’s right and left hands, which are also mirror images that cannot be superimposed.