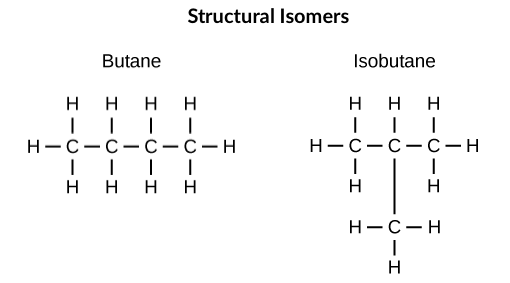
In structural isomers, the atoms in each isomer are connected, or bonded, in different ways. As a result, structural isomers often contain different functional groups or patterns of bonding. Consider butane and isobutane, shown above: both molecules have four carbons and ten hydrogens (), but butane is linear and isobutane is branched. As a result, the two molecules have different chemical properties (such as lower melting and boiling points for isobutane). Because of these differences, butane is typically used as a fuel for cigarette lighters and torches, whereas isobutane is often employed as a refrigerant or as a propellant in spray cans.